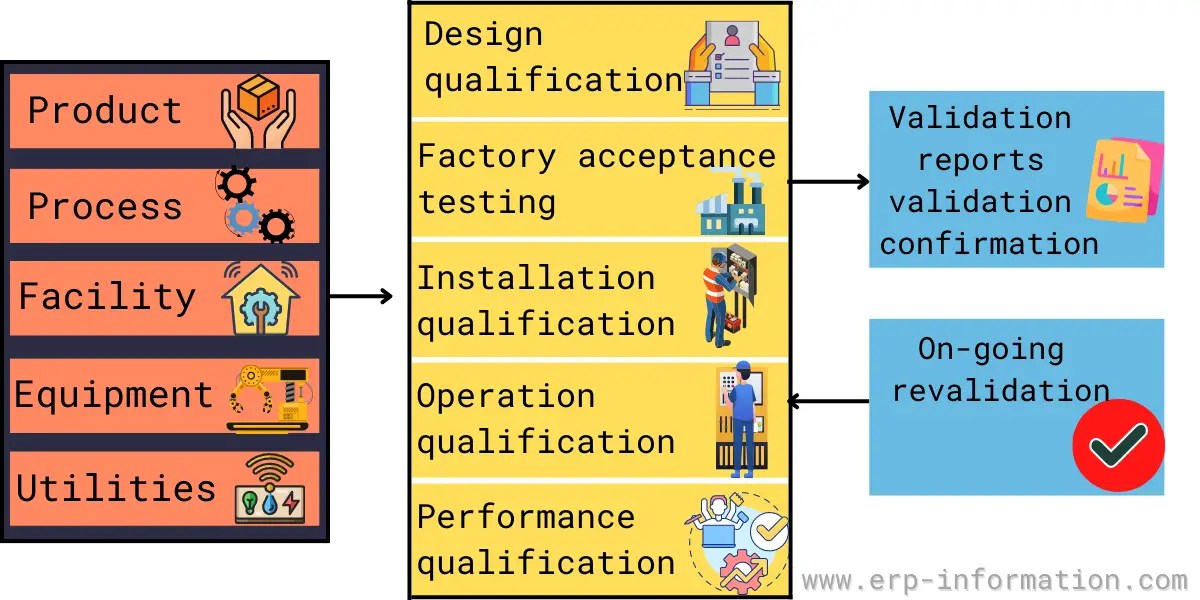
Validation Master Plan Template - The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. The basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and systems. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies.
What is Validation Master Plan? (Template, Examples)
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. The.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. This protocol template provides a comprehensive validation master plan.
Validation Master Plan Template Printable And Enjoyable Learning
A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. You should prepare a validation master plan using templates.
Validation Master Plan Egnyte
The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. The basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu.
Master Validation Plan Template
This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. A validation master plan (vmp) is a documented plan that outlines the overall.
Master Validation Plan Template
Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and systems. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
The basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and systems. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. All.
Validation Master Plan. Understand the importance and benefits.PresentationEZE
All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and systems. The basic principles and application of qualification and validation are.
Validation Master Plan Template Verification And Validation Pharmaceutical
All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. The basic principles and application of qualification and validation are.
FREE 9+ Sample Validation Plan Templates in PDF MS Word
A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and.
A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies. All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. The basic principles and application of qualification and validation are described in annex 15 to the pic/s and eu guide to gmp. You should prepare a validation master plan using templates that cater to each of your facility’s critical processes and systems. Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary.
You Should Prepare A Validation Master Plan Using Templates That Cater To Each Of Your Facility’s Critical Processes And Systems.
Validation master plan (vmp) the vmp serves as the validation roadmap, setting the course, justifying the strategy, out lining the preliminary. A validation master plan (vmp) is a documented plan that outlines the overall strategy and approach for validation activities within a. The master validation plan is designed to provide a planned and systematic framework within which all validation activities will occur. This protocol template provides a comprehensive validation master plan (vmp) protocol for pharmaceutical and medical device companies.
The Basic Principles And Application Of Qualification And Validation Are Described In Annex 15 To The Pic/S And Eu Guide To Gmp.
All of these validation plan templates enable you to write down the entire findings, planning, and expected outcomes at the beginning of the validation project to ensure that the final.